Areas of Interest
Dermatology
CHRONIC VENOUS INSUFFICIENCY (CVI)
CVI is caused by inflammation and blood pooling in the lower extremities leading to varicose veins, swollen legs, stasis dermatitis and eventually venous ulcers.
Prevalance in USA
7.4M
Clinical Stage
PHASE 1/2
CEAP Classification
Internationally Accepted Classification for Chronic Venous Disease

Rising obesity and the aging population are increasing the prevalence of CVI. Current therapies include life style changes (e.g. increased hydration, dietary change), compression therapy (e.g. compression socks), antibiotics to treat skin infections, anti-coagulants to prevent blood clots, and surgery in acute cases. Other than surgery on ulcers, there is no direct treatment available for CVI. Symptoms include:
- Achy or tired legs
- Burning, tingling or “pins and needles” sensation in your legs
- Cramping in your legs at night
- Discolored skin that looks reddish-brown
- Lower led edema
- Flaking or itching skin in the legs and feet
- Full or heavy feeling in your legs.
- Leathery-looking skin on your legs
- Chronic venous ulcers
Untreated CVI can also lead to life threatening deep vein thrombosis (DVT).
AN 8-10% REDUCTION IN LEG VOLUME COULD RESOLVE
THE MAJORITY OF EDEMA CAUSING PAIN, IMPAIRED MOBILITY & Stasis dermatitis

BASELINE (Before)
Dermatitis (Red)
Edema (Swelling)
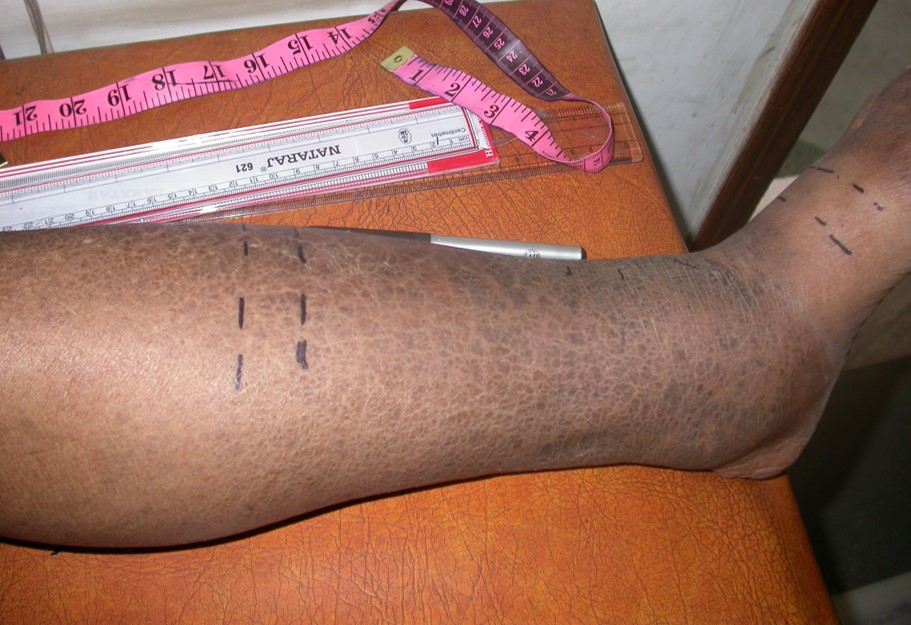
2 WEEKS (After)
Dermatitis is resolved with a 9.75% average reduction in leg volume
Livionex has the potential to be
The First Approved CVI treatment
Ready to initiate Phase 1/2
Double-blind study showed significant improvement in Stage 3 and Stage 4 CVI within 2 weeks
Reducing lower leg edema and resolving stasis dermatitis

