Areas of Interest
CATARACTS
Cataracts are calcium lipid/protein aggregates that increase the opacity of the human lens and lead to impaired vision. It is the #1 cause of world blindness and 99% of all adults over 50 years of age, will get cataracts in their lifetime. It is the single largest line item in US Medicare budget. The current standard of care is surgery, typically reimbursed only for late-stage cataracts.
Diagnosed in USA
27.8M
Clinical Phase
PHASE 2
COMPLETED
Opacity of the lens in early to moderate stage cataracts impairs vision significantly. Patients often see blinding lights and have foggy vision such as in the picture to the left.
#1
leading Cause of blindness globally
Cataract based vision loss starts around age 40, and the average age of surgery is 72 with vision loss increasing with each year. Typical symptoms of pre-surgical cataracts include significantly reduced vision especially in dim light, loss of color perception, and glare.
REFERENCE: PMID 39098755
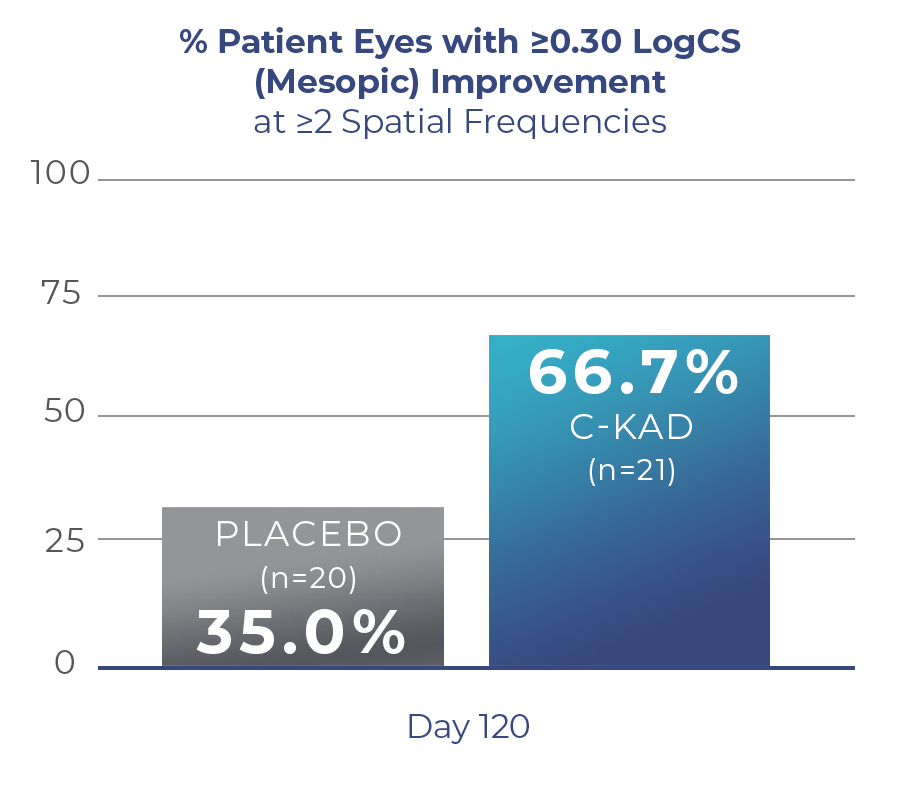
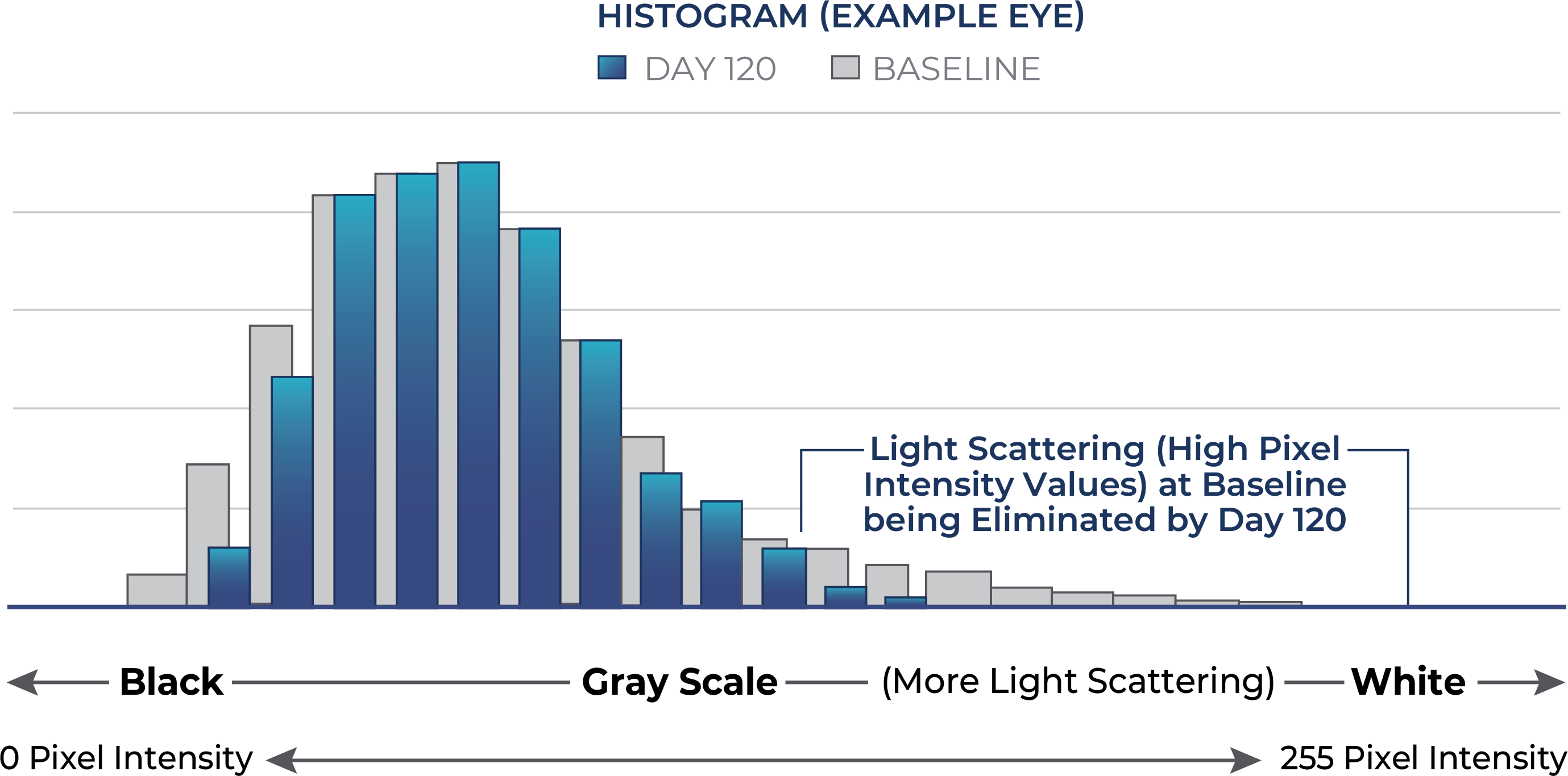
Reduced Average Lens Density with C-KAD
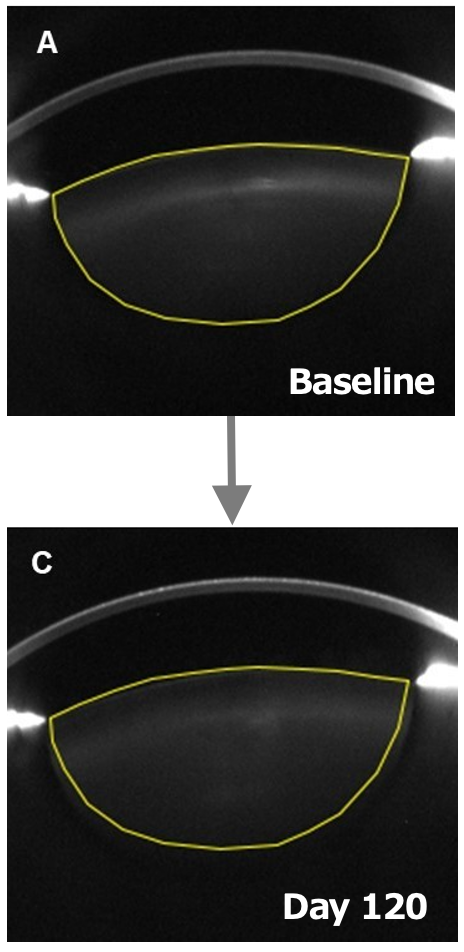
eye drops target pre-surgical cataracts
(between 40 – 72 years)

Sub-analysis of a US Phase I/II trial in early-to-moderate cataracts showed that Livionex C-KAD Eye Drops showed improved mesopic (low light) contrast sensitivity and improved lens clarity.
Finalizing Phase 3 Trials for the cataract indication in consultation with the FDA
In vitro and in vivo pre-clinical data shows that the eye drop may have efficacy in other therapeutic areas including diabetic retinopathy and optic nerve protection.
BAND KERATOPATHY
Band Keratopathy is a calcific band in the cornea that causes extreme discomfort, pain, and impaired vision. Current standard of care is surgical debridement of the corneal epithelium, then using EDTA to remove the calcific deposits in the Bowman’s layer.
Annual Incidence in USA
< 100K
Clinical Phase
PHASE 3
The image on the left displays a band-shaped, horizontal opacity that has grown from the peripheral cornea towards the central cornea.
FASTER APPROVALS
AN APPROVAL IN BAND KERATOPATHY WOULD LEAD TO EXPEDITED NDAs IN OTHER OCULAR INDICATIONS.
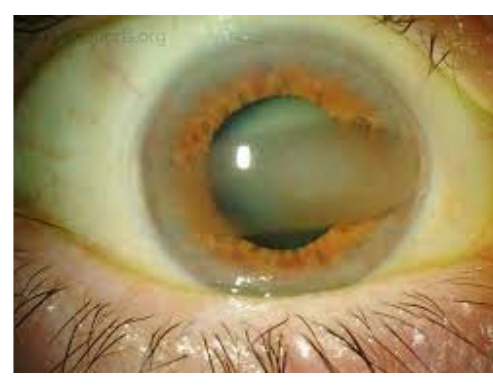
The image above displays a band-shaped, horizontal opacity that has grown from the peripheral cornea towards the central cornea.
FASTER APPROVALS
AN APPROVAL IN BAND KERATOPATHY WOULD LEAD TO EXPEDITED NDAs IN OTHER OCULAR INDICATIONS.
